Copper(II) and Nickel(II) Complexes Derived from Isostructural Bromo- and Fluoro-Containing Bis-Schiff Bases: Syntheses, Crystal Structures and Antimicrobial Activity
DOI:
https://doi.org/10.17344/acsi.2023.8359Abstract
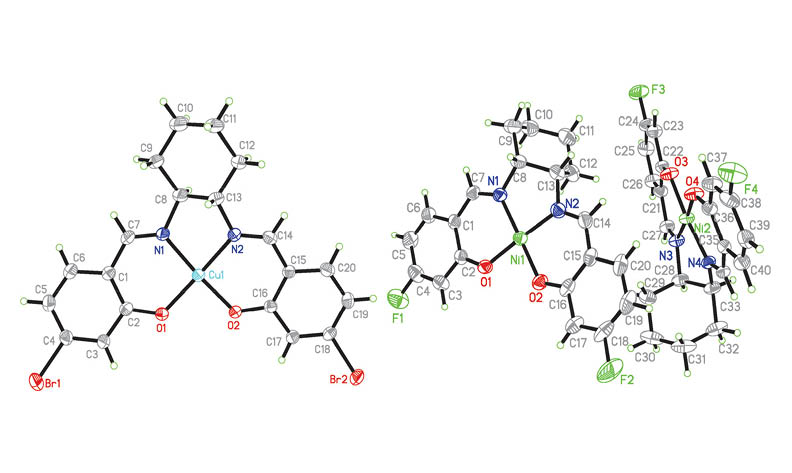
A mononuclear copper(II) complex [CuLa] (1), and three mononuclear nickel(II) complexes [NiLa] (2), [NiLa]·CH3OH (2·CH3OH) and [NiLb] (3), where La and Lb are the dianionic form of N,N'-bis(4-bromosalicylidene)-1,2-cyclohexanediamine (H2La) and N,N'-bis(4-fluorosalicylidene)-1,2-cyclohexanediamine (H2Lb), respectively, were prepared and structurally characterized by spectroscopy method and elemental analyses. The detailed structures were determined by X-ray single crystal diffraction. All the copper and nickel complexes are mononuclear compounds. The metal ions in the complexes are in square planar coordination, with the two phenolate oxygens and two imine nitrogens of the Schiff base ligands. The biological effect of the four complexes were assayed on the bacteria strains Staphylococcus aureus, Escherichia coli and Candida albicans.
Downloads
Published
Issue
Section
License
Copyright (c) 1970 Ke-Sheng Cao, Ling-Wei Xue, Qiao-Ru Liu

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
