Study on the Equilibria of the Complex Formation of Anionic Chelate of Zn(II) with Tridentate Ligand and the Cation of 3-(2-naphtyl)-2,5-diphenyl-2H-tetrazolium chloride
DOI:
https://doi.org/10.17344/acsi.2023.8197Abstract
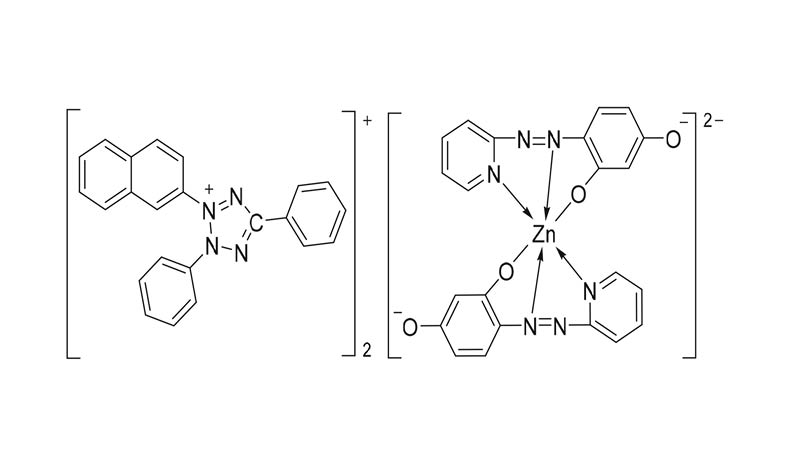
The equilibria of the complex formation between the anionic chelate of zinc(II) with the tridentate ligand of 4-(2-pyridylazo)resorcinol (PAR) and the bulky organic tetrazolium cation of 3-(2-naphtyl)-2,5-diphenyl-2H-tetrazolium chloride (TV) in the liquid-liquid extraction system Zn(II)‒PAR‒TV‒H2O‒2-methyl-1-propanol was studied by spectrophotometric method. The molar ration of the reagents was determined by independent methods under the optimum condition for ion-association and for extraction. The validity of Beer’s law was checked and some analytical characteristics were calculated. The constants, describing the association process in aqueous phase and the extraction equilibria, were calculated. Based on this, a reaction scheme, a general formula and a structural formula of the complex were suggested. The zinc(II) cation is six coordinated with the tridentate ligand through the following atoms: the azo nitrogen, the pyridine nitrogen and the oxygen atom from the phenolic group, which is in ortho position relative to the azo group, each of them forming two five-membered chelate rings.
Downloads
Published
Issue
Section
License
Copyright (c) 1970 Kirila Stojnova, Pavel Yanev, Vanya Lekova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
