First N-allyl-aminothiadiazole Copper(I) π-Complexes: Synthesis and Structural Peculiarities of [Cu(L)CF3SO3] and [Cu2(L)2(H2O)2](SiF6) • 2.5H2O Compounds...
Keywords:
Thiadiazole, copper(I), π-complex, crystal structure, alternating-current synthesisAbstract
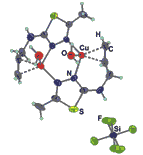
By means of alternating current electrochemical technique two crystalline copper(I) π-complexes with fluorine containing anions [Cu(L)CF3SO3] (1) and [Cu2(L)2(H2O)2](SiF6)2 · 2.5H2O (2) (L – 2-(allyl)-amino-5-methyl-1,3,4-thiadiazole)have been obtained and characterized by X-ray single crystal diffraction and Raman spectroscopy. In both structures the organic molecule L acts as chelate-bridging tridentate ligand being connected to copper(I) by two N atoms of thiadiazole ring and C=C bond from allyl group resulting in a formation of stable cationic dimers [{Cu(L)}2]2+. In the structure 1 oxygen atom from triflate-anion occupies an apical position of the metal coordination polyhedron, while in 2 located far from the metal centre hexafluorosilicate anion allows an appearance of the H2O molecule in copper environment. Hydrogen bonds (D)-H···A (where D = O, N, C; A = O, F) play a significant role in formation of 2D- (1) and 3D- (2) frameworks.
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
