Novel Thiazolactone Derivatives: Synthesis and Quantum Chemical Study
DOI:
https://doi.org/10.17344/acsi.2022.7918Keywords:
Thiazolactone, Erlenmeyer Plöchl reaction, amino acid, DFT studyAbstract
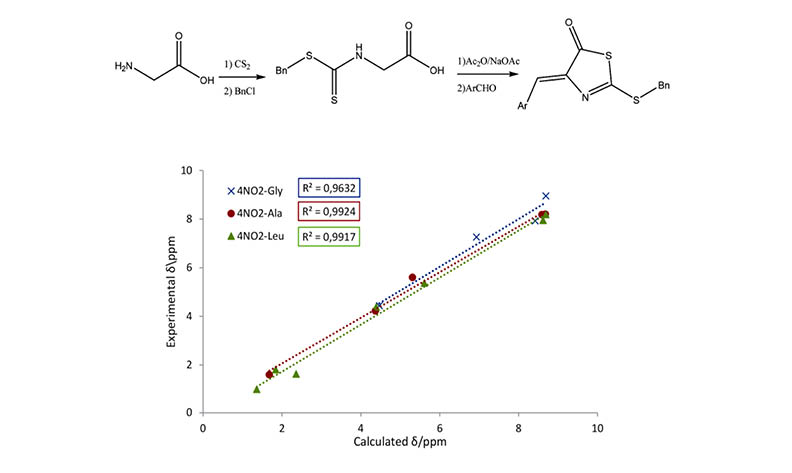
In this research, fifteen novel derivatives of the thiazolacton skeleton were synthesized using Erlenmeyer Plöchl reaction procedure. Glycine, alanine and leucine amino acids are used to make dithiocarbamate precursor by reacting aminoacids with carbon disulfide and benzyl chloride. Obtained benzyl dithiocarbamate undergoes thiazolactone formation in presence of acetic anhydride and then condensed with arylglyoxals as condensing carbonyl group source. Products were characterized using their spectroscopic IR, 1H NMR and 13C NMR data. In continue computational chemistry methods used to get some information about the products such as structural characteristics, charge distribution, 1H NMR and UV-Visible spectra. Results showed that the calculated chemical shifts are in good agreement with experimentally recorded ones. The B3LYP density functional method in conjunction with 6-311++G(d,p) basis set is used for all calculations.
Downloads
Published
Issue
Section
License
Copyright (c) 1970 Behzad Khalili, Niloofar Bakhshi Boroon

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
