Strong Cationic Oxidizers: Thermal Decomposition, Electronic Structure and Magnetism of Their Compounds
Keywords:
Electronic structure, fluorides, magnetism, oxides, oxidizer, silverAbstract
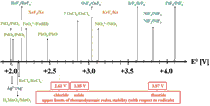
Strong oxidizers could be provisionally defined as compounds for which the standard redox potential exceeds 2.0 V in the NHE scale. Compounds which contain transition or post-transition metals at their unusually high positive oxidation states constitute one important family of strong oxidizers. Majority of such systems typically exhibit either diamagnetic or 'simple’ paramagnetic properties down to very low temperatures. This is connected with the fact that highest oxidation states of metals are stabilized in fluoride environment and that binary high-valence metal fluorides form either molecular (0D) or low-dimensional (usually 1D) crystals. The ternary and higher fluorides are usually 0D in electronic sense leading again to low ordering temperatures. The situation becomes more interesting in selected compounds of Ag(II), the strongest oxidizer among all divalent cations, where one finds 2D or even 3D magnetic ordering at elevated temperatures.
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
