Со(ІІ) AND Ni(II) REMOVAL FROM AQUEOUS SOLUTIONS BY POLYMER AND POLYMER/SILICA ADSORBENTS WITH SULFO AND CARBOXYLIC GROUPS
DOI:
https://doi.org/10.17344/acsi.2022.7819Keywords:
polymer/silica membrane, adsorbent, wastewater treatmentAbstract
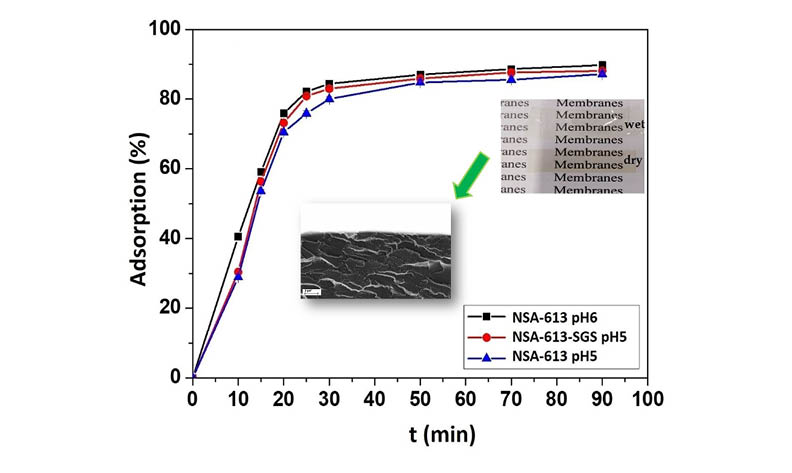
Sulfo and carboxylic groups-containing polymer and polymer/silica membranes poly(acrylonitrile-co-2-acrylamido-2-methylpropane-1-sulfonic acid-co-acrylic acid)[poly(AN-co-AMPS-co-AA)] and poly(AN-co-AMPS-co-AA)/SiO2 were synthesized by UV-initiated polymerization or simultaneous UV-initiated polymerization and sol-gel process in situ and employed as adsorbents for the removal of Co(II) and Ni(II) ions from the aqueous solutions. The adsorption capacity and the effect of pH in the removal process have been studied.
Poly(AN-co-AMPS-co-AA) adsorbent exhibited high efficiency: up to 91,8 % removal of Co(II) and 89,7 % removal of Ni(II). Polymer/silica membranes showed higher removal capacity as compared to polymer ones. The adsorption kinetics of Co(II) and Ni(II) ions were found to be satisfactorily described by the pseudo-second order reaction equation of the Lagergren kinetic model, which suggests the ion-exchange nature of the process.
Downloads
Published
09.09.2023
Issue
Section
Materials science
License
Copyright (c) 1970 Oksana Demchyna

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
