Effect of an anionic surfactant on electrochemical response of Leuprolide used in the treatment of precocious puberty: A reliable voltammetric method
DOI:
https://doi.org/10.17344/acsi.2021.7194Keywords:
Leuprolide acetate, voltammetry, determination sodium dodecyl sulfate, validationAbstract
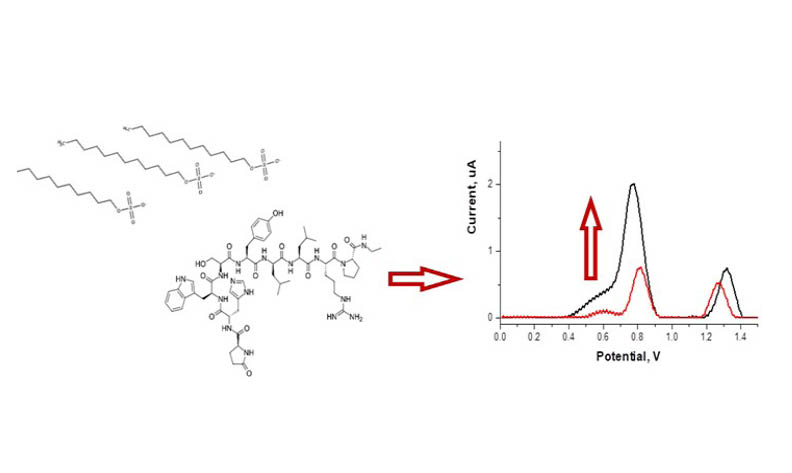
In this study, the rapid, simple and sensitive voltammetric method has been developed for the quantification of leuprolide acetate in the presence of anionic surfactant on a glassy carbon electrode. The effect of surfactant types, pH of the medium, scan rate on the voltammetric response of leuprolide acetate were evaluated. The oxidation peak currents of leuprolide acetate were enhanced in the presence of 8.0 x 10-5 M sodium dodecyl sulfate. The monomer structure of sodium dodecyl sulfate can be attracted to amino moieties in drug structure via the electrostatic interaction. The voltammetric behavior of leuprolide acetate exhibited irreversible and diffusion-adsorption mix controlled processes by cyclic voltammetry. Under optimized conditions, the proposed method exhibits linearity in the concentration range of 3.64 x 10-9 – 2.00 x 10-7 M with a nano-level detection limit of 4.70 x 10-10 M by adsorptive stripping square wave voltammetry. The developed method was applied to an injectable dosage form for the determination of leuprolide acetate with satisfactory accuracy and precision.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Begüm Evranos Aksöz, BURCU DOGAN-TOPAL

This work is licensed under a Creative Commons Attribution 4.0 International License.
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
