Synthesis, Characterization and X-ray Structure of the Adducts of Bis(O-butyldithiocarbonato)nickel(II) with Substituted Pyridines
Keywords:
Adducts, Xanthates (dithiocarbonates), Octahedrally coordinated, direct methods, crystal structure, π– π and C-H••• π interactions.Abstract
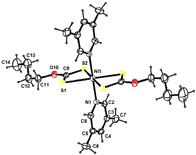
Some mixed ligand complexes of Ni(II) with O-butyldithiocarbonate as a primary ligand and substituted pyridines as secondary ligands have been isolated and characterized on the basis of analytical data, molar conductance, magnetic susceptibility, electronic and infrared spectral studies. The molar conductance studies show their non-electrolytic behavior. Magnetic and electronic spectral studies suggest octahedral stereochemistry around Ni(II) ions. Infrared spectral studies suggest bidentate chelating behavior of O-butyldithiocarbonate monoanion while other ligands show unidentate behavior in their complexes. One of the adduct bis(O-butyldithiocarbonato)bis(3,5-dimethylpyridine)nickel(II) crystallizes in the monoclinic space group P21/c with unit cell parameters. The crystal structure has been solved by direct methods and refined by full matrix least-squares procedures to a final R-value of 0.0379 for 2460 observed reflections. The Ni2+ ion is in a octahedral coordination environment formed by an N2S4 donor set, defined by two chelating dithiocarbonate anions as well as two 3,5-dimethylpyridine ligands with the Ni2+ ion located at the inversion centre. The packing of layers of molecules is stabilized by weak pi-pi and C-H---pi interactions.
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
