New Approached for the Synthesis and Ctytotoxicity of Thiazoles Derived from Cyclohexanone
Keywords:
cyclohexanone, thiophene, thiazole, phenylazoAbstract
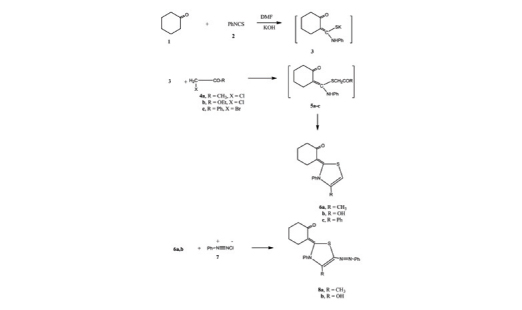
The aim of this work was to synthesis a new class of substituted thiazole derivatives which showed promising anticancer activity. The reaction of cyclohexanone with phenylisothiocyanate gave the thiazole derivatives 6a-c with good yield. The latter compounds reacted with benzendiazonium chloride to form corresponding 5-phenylazothiazole derivatives 8a and 8b, respectively. Moreover, the reaction of thiazole derivatives 6a-c with each of elemental sulfur and either of malononitrile or ethyl cyanoacetate gave the thiophene derivatives 10a-e, respectively. Compounds 10a-e were subjected to a series of heterocylclization reactions to give heterocyclic derivatives. Their cytotoxicity against the four human tumor cells lines was measured and showed promising anticancer activity.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
