Theoretical considerations regarding the thione-thiol tautomerism in 2-(5-mercapto-1,3,4-thiadiazol-2-ylthio)acetic acid
DOI:
https://doi.org/10.17344/acsi.2014.567Keywords:
thiadiazole, pKa, thiol-thione tautomerism, theoretical computations, DFTAbstract
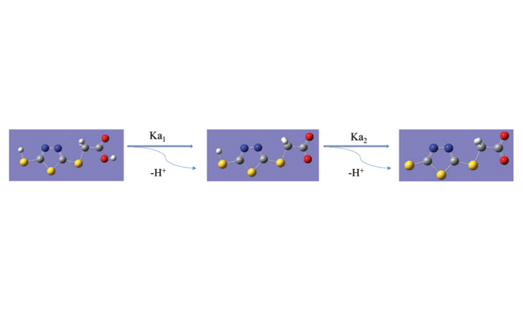
The acidity constants Ka1 and Ka2 of 2-(5-mercapto-1,3,4-thiadiazol-2-ylthio)acetic acid have been determined both by experimental and theoretical methods. pKa computations at B3LYP/6-311+G(d,p) level of theory were carried out for the two tautomeric forms, thiol and thione, of the above-mentioned acid. Comparisons between the experimental and theoretical values led to the establishing of the most stable tautomer of 2-(5-mercapto-1,3,4-thiadiazol-2-ylthio)acetic acid in aqueous solution. Also, a DFT study regarding the reactivity, aromaticity and population analysis of the two tautomers has been performed.
Additional Files
Published
06.02.2015
Issue
Section
Physical chemistry
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
