Solvent Extraction of Calcium and Strontium into Nitrobenzene by Using a Synergistic Mixture of Hydrogen Dicarbollylcobaltate and Bis(Diphenylphosphino)Methane Dioxide
Keywords:
Calcium, strontium, bis(diphenylphosphino)methane dioxide, hydrogen dicarbollylcobaltate, complexation, extraction and stability constants, water-nitrobenzene systemAbstract
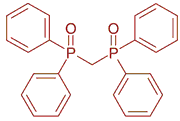
Extraction of microamounts of calcium and strontium by a nitrobenzene solution of hydrogen dicarbollylcobaltate (H+B-) in the presence of bis(diphenylphosphino)methane dioxide (DPPMDO, L) has been investigated. The equilibrium data have been explained assuming that the species HL+, HL2+, ML2(2+), ML3(2+) and ML4(2+) (M2+ = Ca2+, Sr2+) are extracted into the organic phase. The values of extraction and stability constants of the cationic complexes in nitrobenzene saturated with water have been determined. In the considered nitrobenzene medium, it was found that the stability constants of the complexes CaL2(2+), CaL3(2+) and CaL4(2+), where L is DPPMDO, are somewhat higher than those of the corresponding complex species SrL2(2+), SrL3(2+) and SrL4(2+) with the same ligand L.
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
