Complexation of the Ammonium Cation with Dibenzo-18-crown-6: Extraction and DFT Study
Keywords:
Ammonium cation, dibenzo-18-crown-6, complexation, extraction and stability constants, water-nitrobenzene system, DFT, complex structureAbstract
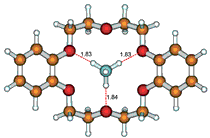
From extraction experiments and gamma-activity measurements, the extraction constan corresponding to the equilibrium NH4(+)(aq) + 1*Na(+)(nb) <=> 1*NH4(+)(nb) + Na(+)(aq) taking place in the two-phase water - nitrobenzene system (1 = dibenzo-18-crown-6, aq = aqueous phase, nb = nitrobenzene phase) was evaluated as log K(ex) (NH4(+),1*Na(+)) = -0.1 +/- 0.1. Further, the stability constant of the 1*NH4(+) complex species in water-saturated nitrobenzene was calculated for a temperature 25 degrees C as log beta (1*NH4(+)) = 5.7 +/- 0.2. Finally, by using quantum mechanical DFT calculations, the most probable structure of the 1*NH4(+) cationic complex was derived. In this complex, the "central" cation NH4(+) is bound by three strong linear hydrogen bonds to the three corresponding ethereal oxygen atoms of the parent crown ligand 1. The interaction energy of the resulting complex 1*NH4(+) was found to be -796.1 kJ/mol, confirming the formation of the considered complex species.
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
