Fused 1,5-benzothiazepines from o-aminothiophenol and its derivatives as versatile synthons
Keywords:
o-aminothiophenol, chalcones, cyclocondensation, green synthesis, 1, 3-dipolar cycloadditionsAbstract
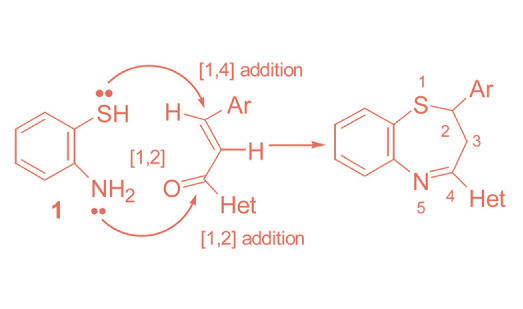
This review describes the reactions of o-aminothiophenol and its derivatives as building blocks for the synthesis of polyfunctionalised 1,5-benzothiazipines with pharmacological interest. Annelated 1,5-benzothiazipines were prepared by a cyclocondensation reaction of o-aminothiophenol and its derivatives with carbonyl and other functionalities. In case of carbonyl function this reaction takes place by a nucleophilic addition, followed by cyclisation concomitant with elimination of water. The objective of this survey is to provide a comprehensive account of the synthesis of various 1,5-benzothiazepines derivatives and their potential to develop better chemotherapeutic agents.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
