The formation of passive layers on zinc based platings
DOI:
https://doi.org/10.17344/acsi.2016.2690Keywords:
Alloy coatings, zinc, EIS, electrodeposition, passivation, corrosion protectionAbstract
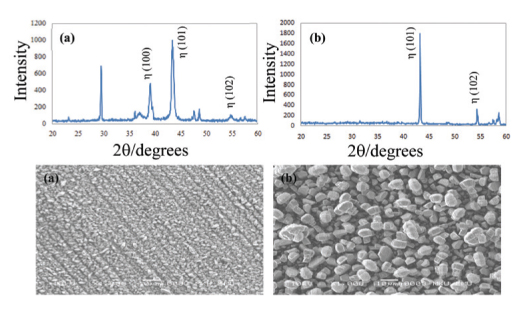
Zinc-iron (ZnFe) and zinc-iron-cobalt (ZnFeCo) platings were achieved on carbon steel applying 3 mA current values. Then, oxalate (OX) and tartrate (Tart) passive layers obtained in sodium oxalate and sodium tartrate medium were formed on carbon steel, ZnFe and ZnFeCo plated carbon steel. SEM images showed that the passive layers on CS, CS/ZnFe and CS/ZnFeCo electrodes exhibited different crystal structures. Corrosion tests revealed that the ZnFeCo particles provided a significant barrier efficiency on CS layer when compared with ZnFe alloy plating. Furthermore, OX layers on ZnFe and ZnFeCo plated carbon steel electrodes exhibited better physical barrier behavior on than those of Tart layers in longer periods
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
