Synthesis of 6-N-R-Tetrazolo[1,5-c]quinazolin-5(6H)-ones and Their Anticancer Activity
DOI:
https://doi.org/10.17344/acsi.2016.2464Keywords:
anticancer, 6-N-R-tetrazolo[1, 5-c]quinazolin-5(6H)-ones, models, QSAR, synthesisAbstract
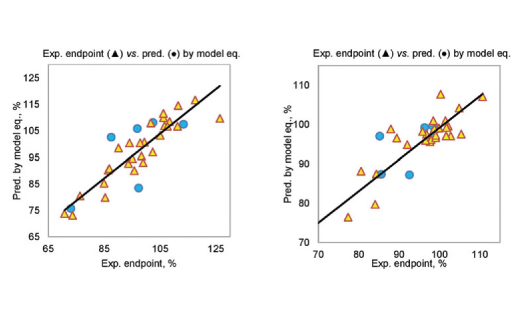
Chemical compounds with tetrazolo ring are very interesting systems that can be valuable in pharmaceutical and clinical applications, especially as anticancer agents. In this work, novel 6-N-R-tetrazolo[1,5-c]quinazolin-5(6H)-ones were synthesized. A large set of IR, LC-, EI-MS, 1H, 13C NMR and elemental analysis data are collected and evaluated for their structures and purity. Details of synthesis, namely the N-alkylation, were discussed, including reaction with secondary and tertiary amides. Based on available experimental data eight QSAR-models for anticancer activity were developed. In addition, four new synthesized compounds (2.7, 3.2, 5.2, 5.3) were tested in vitro for anticancer activity at 10 μM against 60 cell lines of nine different cancer types: leukemia, melanoma, lung, colon, CNS, ovarian, renal, prostate, and breast cancers. The obtained experimental data correspond to QSAR-models results. Further synthesis of substances within series of substituted tetrazolo[1,5-c]quinazoline systems will be arranged based on suggestions from QSAR-models to develop improved compounds with better anticancer activity.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
