Study of Thermodynamic Properties of Solution of Ampicillin Sodium in Methanol at T = 298.15 K
Keywords:
Antibiotic, Osmotic coefficient, Vapor pressure, Thermodynamic modelsAbstract
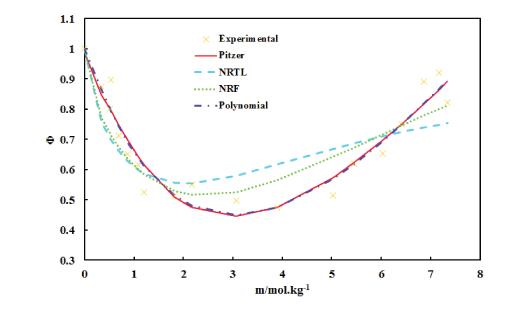
Osmotic coefficients of the solution of Ampicillin sodium in methanol at T = 298.15 K were measured using the isopiestic technique and head space-gas chromatography. The experimental osmotic coefficients have been correlated using the ion interaction model of Pitzer, e-NRTL model of Chen, NRF and a fourth– order polynomial in terms of molality. The vapor pressures of the solutions and the solvent activities have been calculated from the osmotic coefficients. Reliability of the models in expression of the osmotic coefficients were compared on the basis of standard deviation of the fittings.
Downloads
Additional Files
Published
11.07.2014
Issue
Section
Physical chemistry
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
