A Study of Donor-Acceptor in the Charge Transfer Molecular Complexes of Some Thiacrown Ethers with Dihalogen Molecules by DFT Method
Keywords:
Donor-acceptor, charge transfer, thiacrown ether, natural charges, density functional theoryAbstract
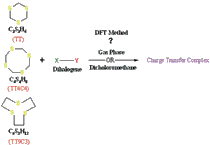
The molecular complexes of 1,3,5-trithiane, (TT), tetrathia-8-crown-4, (TT8C4), and trithia-9-crown-3 , (TT9C3) with dihalogens in the ground state were investigated in the gas and dicholoromethane phases using B3LYP method and 6-31G** and 6-31+G** bases sets. In both TT and TT8C4 complexes, it is predicted that charge transfer takes place from the dihalogen to the thiacrown ether molecule; the magnitude trend of the total CT was ICl > IBr > I2, and Cl2 > Br2 > I2, respectively. There was not such a trend with TT9C3. The frequency analysis showed that all complexes in the excited state were unstable. The analysis of natural bond orbitals and comparison of the calculated thermodynamic quantities of the complexes between the gas phase and tetrachloromethane solution confirmed the results.
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
