Reductive Heck Reactions and [3+2] Cycloadditions of Unsaturated N,N’-Bistricyclic Imides
Keywords:
Alkenes, cycloadditions, heterocycles, hydroarylations, triyclic imidesAbstract
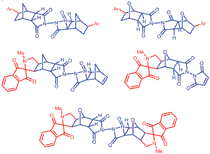
The C-C coupling of N,N'-bis(5-norbornene-2,3-dicarboximide) (3) and N,N'-bis(7-oxa-5-norbornene-2,3-dicarboximide) (6) with aryl and heteroaryl iodides gave under reductive Heck conditions the C-aryl(hetaryl), substituted N,N'-bistricyclic imides 7a-f and 8a,b. The fused spiro-1,3-indandionolylpyrrolidine compounds, 9, 10 and 11 were also obtained from ninhydrine, sarcosine and 3 or 6 via [3+2]cycloaddition.
Downloads
Published
22.10.2013
Issue
Section
Organic chemistry
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
