The Binding Sites of Cadmium to a Reduced Form of Glutathione
Keywords:
Glutathione, cadmium complex, vibrational spectroscopy, NMR spectroscopyAbstract
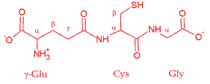
Glutathione is the most abundant low molecular weight thiol-containing molecule in biological cells with a strong tendency to interact with metal ions. Among the eight possible glutathione binding sites, only two are determined as groups that interact with the Cd2+ ion. Analysis of vibrational spectra and 13C and 1H NMR spectra revealed that thiol and glutamyl's carboxylic groups are groups that cooperate in interaction with Cd2+ ions. The coordination of Cd2+ with those groups was supported by the application of auxiliary molecules (D-penicillamine, glycine, cysteine and glutamic acid dipeptides, mercaptosuccinic acid and N-acetyl-L-cysteine). These molecules provide a reliable assignment of the fundamental vibrations in the glutathione vibrational spectra. Concentration-dependent measurements of Cd2+ ions showed that the optimal stoichiometry of coordination with the glutathione molecule is 1:1. The analysis of 3J (Halpha, H(N)) coupling constants and conformational sensitive bands in the glutathione vibrational spectra suggest that interaction with Cd2+ ions significantly alters glutathione backbone conformation. The binding of ions induced the conformational change of the cysteine backbone from a predominantly beta structure to P(II).
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
