Extraction and DFT Study on the Complexation of Zn2+ with Beauvericin
Keywords:
Beauvericin, zinc cation, complexation, extraction and stability constants, water – nitrobenzene system, DFT, complex structureAbstract
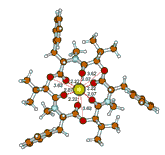
From extraction experiments and γ-activity measurements, the exchange extraction constant corresponding to the equilibrium Zn2+(aq) + 1 . Sr2+(nb) ⇔1 . Zn2+(nb) + Sr2+(aq) taking place in the two–phase water–nitrobenzene system (1 = beauvericin; aq = aqueous phase, nb = nitrobenzene phase) was evaluated as log Kex (Zn2+, 1 . Sr2+) = –0.3 ± 0.1. Further, the stability constant of the beauvericin – zinc complex (abbrev. 1 . Zn2+) in nitrobenzene saturated with water was calculated for a temperature of 25 °C: log βnb (1 . Zn2+) = 9.1 ± 0.2. Finally, by using quantum mechanical DFT calculations, the most probable structure of the 1 . Zn2+ complex species was predicted.
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
