Synthesis, Crystal Structures and Urease Inhibition of N'-(2-Bromobenzylidene)-2-(4-nitrophenoxy)acetohydrazide and N'-(4-Nitrobenzylidene)-2-(4-nitrophenoxy)acetohydrazide
DOI:
https://doi.org/10.17344/acsi.2015.1770Keywords:
Hydrazone, Crystal structure, Hydrogen bonds, X-ray diffraction, Urease inhibition.Abstract
Two new hydrazone compounds, N'-(2-bromobenzylidene)-2-(4-nitrophenoxy)acetohydrazide (1) and N'-(4-nitrobenzylidene)-2-(4-nitrophenoxy)acetohydrazide (2), were prepared and structurally characterized by elemental analysis, IR, UV-Vis and 1H NMR spectroscopy, and single-crystal X-ray diffraction. Compound 1 crystallizes in the monoclinic space group P21/n with unit cell dimensions of a = 5.3064(5) Å, b = 18.202(2) Å, c = 15.970(2) Å, β = 95.866(3)º, V = 1534.4(2) Å3, Z = 4, R1 = 0.0457, and wR2 = 0.0975. Compound 2 crystallizes in the monoclinic space group P21/c with unit cell dimensions of a = 4.6008(7) Å, b = 14.451(2) Å, c = 23.296(3) Å, β = 93.620(2)º, V = 1545.8(4) Å3, Z = 4, R1 = 0.0441, and wR2 = 0.0985. Structures of the compounds are stabilized by hydrogen bonds and π···π interactions. The urease inhibitory activities of the compounds were studied. Both compounds show strong urease inhibitory activities, with IC50 values of 8.4 and 20.2 μM, respectively.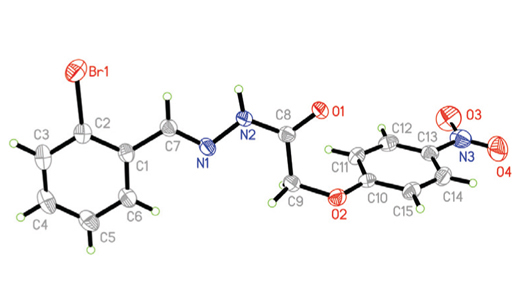
Downloads
Additional Files
Published
16.11.2015
Issue
Section
Organic chemistry
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
