Kinetics and Mechanism of the Ligand Exchange Reaction Between Tetraaza Macrocycle Ligand and Cu(II) Tetradentate Amine-Amide Complexes
DOI:
https://doi.org/10.17344/acsi.2015.1611Keywords:
macrocycle, acyclic, diamine diamide, Cu complexes, ligand exchangeAbstract
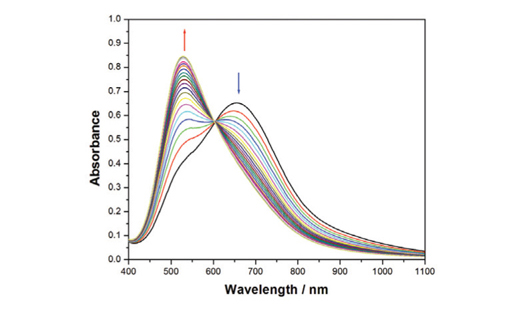
The kinetics of the ligand exchange reaction of tetraaza macrocycle, teazma (teazmais 5,7,7,12,14,14-hexamethyl-1,4,8,11-tetraazacyclotetradeca-4,11-diene dihydrogen perchlorate) with Cu(bcen)2+ and Cu(bctn)2+, where bcen and bctn are N,N'-bis(β-carbamoylethyl) ethylendiamine) and N,N'-bis(β-carbamoylethyl) propylendiamine), respectively, have been studied by visible spectrophotometry in dimethylformamide, DMF, solvent at 25 ± 0.2°C. In the system of Cu(bctn)2+/teazma,the ligand exchange reaction proceeds in a two-step-consecutive manner, with two rate constants k(bctn)(1) and k(bctn)(2). The first reaction step was dependent on the concentration of teazma macrocycle, while the second reaction step was independent. However, it is found that the ligand exchange reaction in Cu(bcen)2+/teazma proceeds in an one-step with the rate constant k(bcen). The rate constant is dependent on [teazma] macrocycle. The ligand exchange reaction in the system of Cu(bcen)2+/teazma is not complete and after some progress, the reaction reaches equilibrium. On the basis of results, a reaction mechanism is proposed and discussed for the ligand exchange rate.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
