Kinetics and Thermodynamics of Adsorption of Fuchsin Acid on Nickel Oxide Nanoparticles
Keywords:
Adsorption, Fuchsin acid, NiO nanoparticle, KASRA modelAbstract
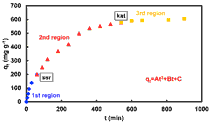
NiO nanoparticle was used to adsorb fuchsin acid (FA) from aqueous solution. In the used concentration range of FA, its adsorption isotherms on NiO nanoparticles were three-region. NiO nanoparticle was prepared via the thermal decomposition of the tris(ethylenediamine)Ni(II) nitrate complex as a new precursor. In this work, effects of temperature, concentration, particle size, shaking rate, contact time, pH of the solution were investigated. Adsorption process was exothermic in the first and second regions and endothermic in the third region. Adsorption kinetics was studied by a number of equations including the KASRA, pseudo-first-order, pseudo-second-order, Elovich, Avrami and pore-diffusion equations. Adsorption acceleration and adsorption velocity values of this process were obtained by the KASRA equation and it was shown that with increase in FA concentration or temperature or shaking rate, initial adsorption velocity values of process increase.
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
