H-Bonded CH 3 SO/H 2 SO 4 /H 2 O Complexes: Quantum Chemical Study
DOI:
https://doi.org/10.17344/acsi.2015.1512Keywords:
Hydrogen-bond complexes, methyl sulfinyl radical, sulfuric acid, water, nucleation precursors, quantum chemical methodsAbstract
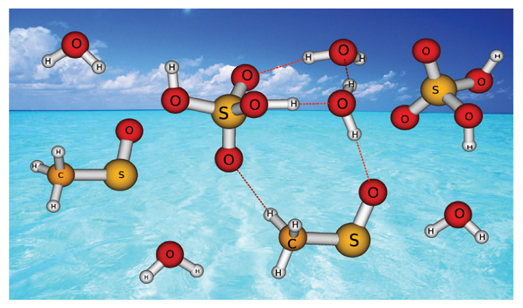
The structural, electronic, and spectroscopic properties of complexes between methyl sulfinyl radical, sulfuric acid and water molecules have been studied by density functional theory and ab initio methods. The hydrogen bond interactions between the CH3SO radical, H2SO4 and H2O molecules have been characterized. Calculations predict relative large binding energies of the complexes being 12.2 kcal mol-1 for the most stable CH3SO-H2SO4 complex, 19.1 kcal mol-1 for CH3SO-H2SO4-H2O complex and 28.8 kcal mol-1 for CH3SO-H2SO4-2H2O complex at CBS-QB3 level of theory. The relatively high stabilization of the complexes is likely to have significant effects on the overall processes that lead to formation of new particles in the atmosphere. Infrared spectroscopy is suggested as a potential useful tool for detection of these complexes either in the laboratory experiments or in the atmospheric observations. The electronic spectra of the complexes have been examined and their photochemical spectral features are discussed. The hydrated CH3SO-H2SO4 complexes can be expected to undergo photolysis in the sunlight.
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
