Reaction between Chromium(III) and EDTA ions: An Overlooked Mechanism of Case Study Reaction of Chemical Kinetics
DOI:
https://doi.org/10.17344/acsi.2015.1492Keywords:
Hexaaquachromium(III) ion, EDTA, UV/Vis spectrophotometry, reaction mechanism, chemical kineticsAbstract
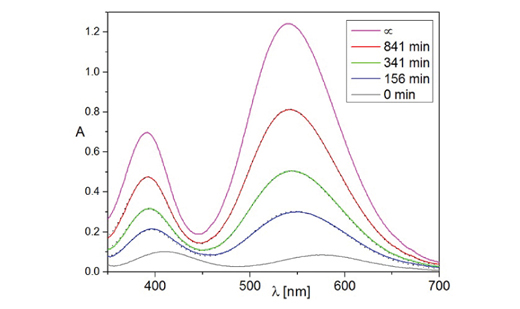
Existing explanations of reaction mechanism of case study reaction of chemical kinetics between Cr(III) ions and ethylenediaminetetraacetic acid (EDTA) contradicts to modern chromium(III) coordination chemistry data. Absorption UV and visible light spectra were therefore recorded during the reaction between aqueous solution of Cr(NO3)3 and EDTA in order to obtain a new insight into this reaction. Analysis of the obtained spectra showed that only very small fraction of intermediates can be present in solution during course of the reaction. The reaction scheme was established and according to it a simplified model calculations carried out using literature data constants when available and adjusted values of their sound estimates when they were missing. Reasonable agreement of model calculations with experimental data was obtained at pH values of 3.8 and 4.5 but the model failed to reproduce measured rate of reaction at pH 5.5, probably due to use of oversimplified model.
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
