Viscosity B-Coefficient for Sodium Chloride in Aqueous Mixtures of 1,4-Dioxane at Different Temperatures
DOI:
https://doi.org/10.17344/acsi.2015.1445Keywords:
Sodium chloride, water–1, 4-dioxane binary mixtures, viscosity data, viscosity B-coefficient, solute ions-solvent interactionsAbstract
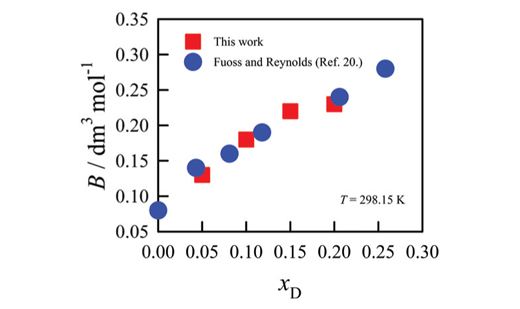
Viscosities of sodium chloride solutions have been measured in water–1,4-dioxane binary mixtures (with 0.05, 0.10, 0.15 and 0.20 mole fractions of dioxane) in the temperature range from 278.15 to 318.15 K. The relative viscosity data have been analyzed and interpreted in terms of the rearranged Jones-Dole equation, (hr – 1) – Ac1/2 = Bc. From the literature limiting ionic conductivity data, the viscosity A-coefficients have been calculated from Falkenhagen and Vernon theory. The viscosity B-coefficients of NaCl are positive at all temperatures for all studied solvents. These data were compared with the data for NaCl in aqueous medium at different temperatures reported in literature. The solute ions-solvent interactions have been discussed.
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
