Effects of Translational and Rotational Degrees of Freedom on the Hydration of Ionic Solutes as Seen by Popular Water Models
DOI:
https://doi.org/10.17344/acsi.2014.1291Keywords:
ionic hydration, water models, degrees of freedom, molecular dynamicsAbstract
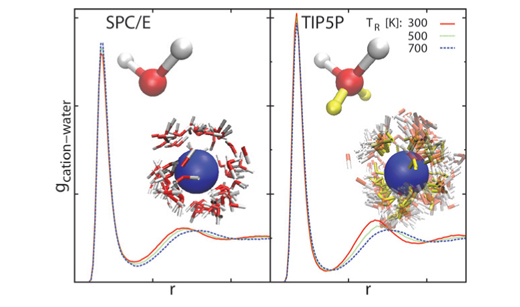
We employed molecular dynamics simulations with separate thermostats for translational and rotational temperatures in order to study the effects of these degrees of freedom on the hydration of ions. In this work we examine how water models, differing in charge distribution, respond to rise of the rotational temperature. The study shows that popular water models can be divided, with respect to the distribution of negative charge, into two groups leading to a different response upon an increase in the rotational temperature. Differences arise in the hydration of cations, as the negative charge distribution on the model solvent represents the determining factor in such cases. The cation-water correlation increases with the increasing rotational temperature when negative charge is placed in (or close to) the centre of the water molecule (a typical example is the SPC water model) and decreases when the negative charge is shifted from its centre (as in the TIP5P model of water). Since all the water models examined here have similar distributions of positive charge, they all exhibit similar trends in solvation of anions upon an increase of rotational temperature. As expected, the effect of translational temperature variation is the same for all water models studied; any increase in translational temperature decreases the solute-water correlations.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
