Optimization and Validation of a Sensitive HPLC–PDA Method for Simultaneous Determination of Torasemide and Spironolactone in Human Plasma using Central Composite Design
DOI:
https://doi.org/10.17344/acsi.2014.1262Keywords:
Column liquid Chromatography, Torasemide, Spironolactone, Human plasma, Bioanalytical method validation, Central Composite DesignAbstract
A sensitive, accurate, precise and rapid HPLC-PDA method was developed and validated for the simultaneous determination of torasemide and spironolactone in human plasma using Design of experiments. Central composite design was used to optimize the method using content of acetonitrile, concentration of buffer and pH of mobile phase as independent variables, while the retention factor of spironolactone, resolution between torasemide and phenobarbitone; and retention time of phenobarbitone were chosen as dependent variables. The chromatographic separation was achieved on phenomenex C18 column and the mobile phase comprising 20 mM potassium dihydrogen ortho phosphate buffer (pH-3.2) and acetonitrile in 82.5:17.5 v/v pumped at a flow rate of 1.0 mL min-1. The method was validated according to USFDA guidelines in terms of selectivity, linearity, accuracy, precision, recovery and stability. The limit of quantitation values were 80 and 50 ng mL-1 for torasemide and spironolactone respectively. Furthermore, the sensitivity and simplicity of the method suggests the validity of method for routine clinical studies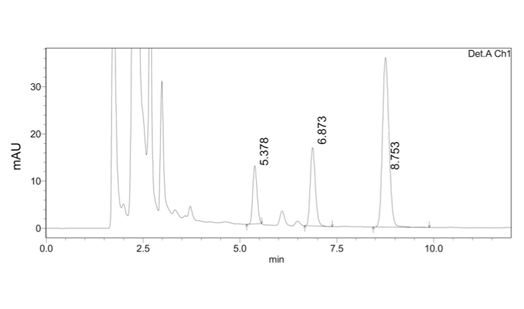
Downloads
Additional Files
Published
27.03.2015
Issue
Section
Analytical chemistry
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
