The role of Ionic surfactants on the solubilization of cyclohexenone Compounds in aqueous media
Keywords:
Cyclohexenone carboxylates, synthesis, Ionic surfactants, solubilization, partitioning studyAbstract
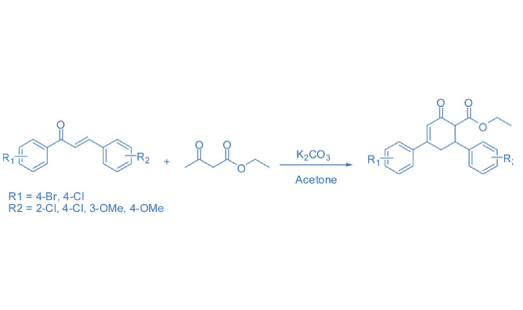
The solubilization and partitioning study of five newly synthesized organic compounds (Cyclohexenone Carboxylates) with ionic surfactants, sodium dodecylsulphate (SDS) and cetyltrimethylammonium bromide (CTAB) was studied using ultraviolet-visible absorption spectroscopy technique. The differential spectroscopic technique was employed to study the partition coefficient (Kx) of organic molecules between bulk water phase and the miceller phase. The values of partitioning coefficient were in the range 29.714×103 to 5.46×106. The standard free energy of partitioning (∆Gop) was also determined, which was found out in the range of -25 to -38 kJ /mole and shows the stability of the system. The results show that the cyclohexenone carboxylate compounds have great interactions with CTAB as compared to SDS.
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
