Syntheses and crystal structures of vanadium and iron chloride complexes with diglyme
DOI:
https://doi.org/10.17344/acsi.2014.1210Keywords:
Iron, Vanadium, Chloride, Di(2-Methoxyethyl), mer-isomer, fac-isomerAbstract
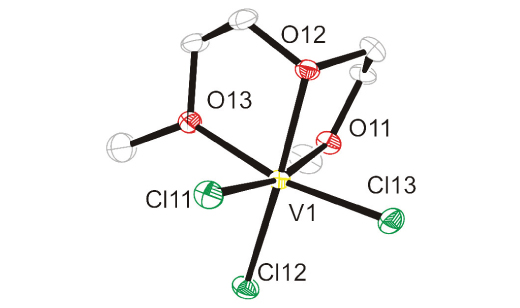
A mononuclear molecular complex fac-[VCl3(diglyme)] (1) resulted from the reaction of VCl3 and diglyme (diglyme = di(2-methoxyethyl)ether) in dichloromethane. The violet complex 1 is a sensitive substance which slowly oxidized to a new, blue mononuclear molecular complex, fac-[VOCl2(diglyme)] (2) in the presence of air.
The synthesis of iron(II), iron(III) complex [FeCl(diglyme)(THF)]2[FeCl4)]2 (3) was achieved by the reaction of yellow-green, partly oxidized FeCl2.4H2O, diglyme and chlorotrimethyl-silane in tetrahydofuran. The compound consists of the dinuclear cations with octahedral environment of iron(II) and the tetrahedral anions of iron(III). A pure iron(II) chloride-diglyme complex [FeCl2(diglyme)]2 (4) was gained by the reaction of freshly prepared iron(II) chloride hydrate, diglyme and chlorotrimethylsilane in dichloromethane. Diglyme is coordinated in the meridional mode to octahedral iron(II) in dinuclear cations of 3 and in dinuclear molecules 4.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
