Mass spectrometric study of colchicine and its synthetic derivatives 10-alkylthiocolchicines
DOI:
https://doi.org/10.17344/acsi.2014.1125Keywords:
colchicine, 10-alkylthiocolchicine derivatives, EI–MS mass spectra, fragmentation pathways, MALDI –TOF MS of colchicine derivativesAbstract
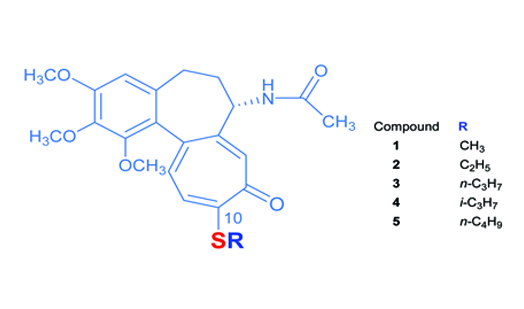
The mass spectrometric behaviour of five 10-alkylthiocolchicine derivatives has been studied by means of electron ionization and MALDI-TOF MS techniques.
The principal electron-induced fragmentation pathways of molecular ions of above colchicine derivatives have been investigated, as well as collision-induced dissociation (CID) MALDI have been used to gain structural information. This paper contains for the first time, to the best of our knowledge, MALDI MS/MS data for colchicine and its alkylthio derivatives. It has been shown also that the data derived from mass spectra can be used in analytics for determination of natural and modified alkaloids of colchicine group. The detailed fragmentation pathways proposed here could aid in the characterization of other colchicines of these type. The utility of different mass spectral methods for analysis of compounds of this class has been evaluated. Due to the cytotoxic activity towards tumour cell lines, 10-alkylthiocolchicines may be considered as the active ingredients of anticancer agent. If these compounds find use in medical treatment, their distribution in organism and metabolism will have to be monitored by spectroscopic or spectrometric methods. The characteristic fragment ions may be used by Selected Reaction Monitoring method for determination of colchicine analogues.
Downloads
Additional Files
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
