Determination of the Interactions Between Zn2+ and Water Soluble Polymer Ligands with Potential Use in Controlled Drug Delivery
Keywords:
Polyamino acid, controlled drug delivery, biodegradable carrier, fluorescence spectroscopy, zinc, polymer coordination compound.Abstract
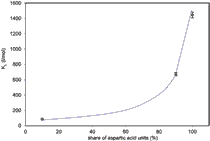
Biodegradable copolymers of aspartic and lactic acids were synthesized for potential use in controlled drug release. The proportion of aspartic acid moieties in the copolymers was 0.9 and 0.1, the molecules were partially branched and had absolute molar masses over 100,000 g/mol. The drug could be attached to the copolymer via metal (particularly zinc) ions, so a method to estimate the interactions between zinc ions and the water-soluble polymers by fluorescence spectroscopy was developed. The stability constants of binding of zinc and the concentrations of zinc bound to polymer were determined. The results confirm that zinc ions at pH 6 preferentially bind to side groups of aspartic acid units of the copolymers.
Downloads
Published
Issue
Section
License
Except where otherwise noted, articles in this journal are published under the Creative Commons Attribution 4.0 International License
